- Get link
- X
- Other Apps
NUBEQA is a drug for the treatment of prostate cancer that has not spread to other parts of the body non-metastatic and no longer responds to a. Για την προβολή της πλήρους καταχώρησης απαιτείται συνδρομή σε ισχύ.
 Frontiers Second Generation Antiandrogens From Discovery To Standard Of Care In Castration Resistant Prostate Cancer Oncology
Frontiers Second Generation Antiandrogens From Discovery To Standard Of Care In Castration Resistant Prostate Cancer Oncology
Αποκτήστε πρόσβαση σε όλες τις πληροφορίες και τα εργαλεία του Galinosgr δωρεάν για.
Nubeqa mechanism of action. 42 Posology and method of administration. Based on its mechanism of action NUBEQA can cause fetal harm and loss of pregnancy when administered to a pregnant female. Nubeqa is taken orally by mouth twice daily with food.
Based on its mechanism of action Nubeqa may cause fetal harm when administered during pregnancy. What is the mechanism of action. There are no data available with the use of Nubeqa during pregnancy in humans.
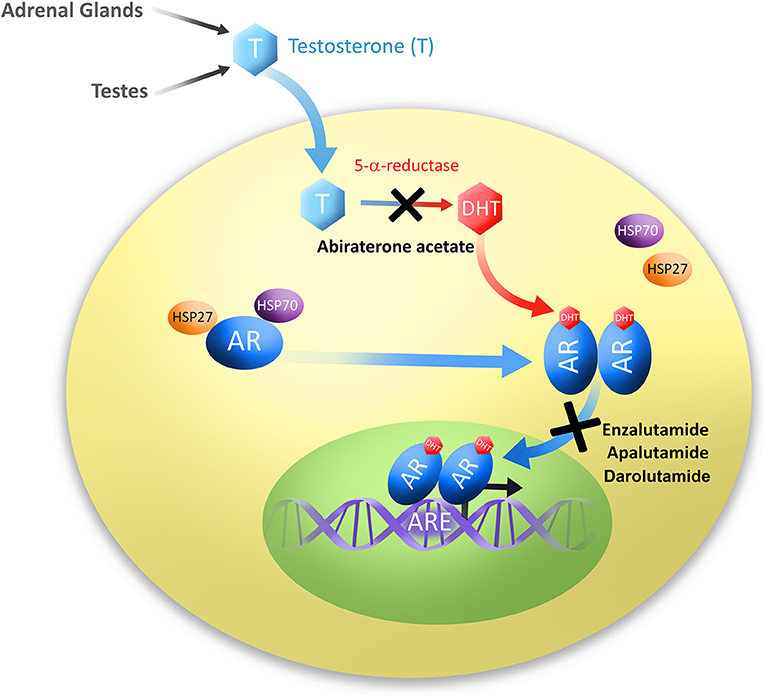
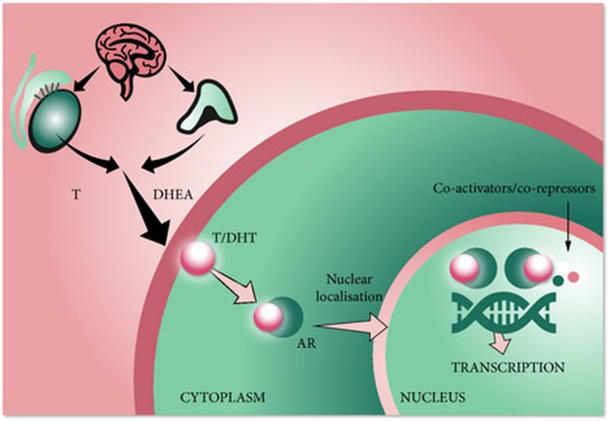
Darolutamide competitively inhibits androgen binding AR nuclear translocation and AR mediated transcription. It is specifically approved to treat non-metastatic castration-resistant prostate cancer in conjunction with surgical or medical castration. Nubeqa is contraindicated in women who are or may become pregnant.
European Medicines Agency EMA for Nubeqa through the centralised procedure falling within the Article 31 and point 3 of Annex of Regulation EC No 726 2004. The mechanism of action of Nubeqa is to block the effects of testosterone. Darolutamide sold under the brand name Nubeqa is an antiandrogen medication which is used in the treatment of non-metastatic castration-resistant prostate cancer in men.
Prostate cancerthat has not metastasizedspread to other parts of the body and is castrationresistanthas not respondedto treatments that lower testosteronelevels. Women of childbearing potential contraception in males and females. NUBEQA is not indicated in women.
Mechanism of action. The eligibility to the centralised procedure was agreed upon by the EMACHMP on 23 March 2017. However based on the mechanism of action NUBEQA can cause embryofetal harm or loss of pregnancy.
Nubeqa - Clinical Pharmacology Mechanism of Action. There are no human data on the use of NUBEQA in pregnant women. Darolutamide competitively inhibits androgen.
Prostate cancer depends on testosterone to grow and spread. Darolutamide is an androgen receptor AR inhibitor with a flexible polar-substituted pyrazole structure that binds with high affinity directly to the receptor ligand binding domain. Based on its mechanism of action NUBEQA can cause fetal harm and loss of pregnancy when administered to a pregnant female see CLINICAL PHARMACOLOGY.
How is Nubeqa given administered. The medication is taken by mouth twice per day with food. Advise males with female partners of reproductive potential to use effective contraception during treatment and for 1 week after the last dose of NUBEQA see Use In Specific Populations.
NUBEQA is indicated for the treatment of adult men with non-metastatic castration resistant prostate cancer nmCRPC who are at high risk of developing metastatic disease see section 51. Darolutamide is approved to treat. Find patient medical information for Nubeqa oral on WebMD including its uses side effects and safety interactions pictures warnings and user ratings.
The way a drug works is called its mechanism of action. Nubeqa interferes with the ability of male hormones to bind to their receptors within a cell and also reduces the ability of the receptors to enter the nucleus and stimulate cell growth. Animal embryo fetal toxicology studies have not been performed.
Darolutamide is also being studied in the treatment of other types of cancer. Based on its mechanism of action Nubeqa may cause fetal harm when administered during pregnancy. The safety and efficacy of NUBEQA have not been established in females.
Based on its mechanism of action NUBEQA can cause fetal harm and loss of pregnancy when administered to a pregnant female see Clinical Pharmacology. There are no data available with the use of Nubeqa during pregnancy in humans. Advise males with female partners of reproductive potential to use effective contraception during treatment and for 1 week after the last dose of NUBEQA see Use in Specific Populations 81.
Darolutamide exposure at 600 mg twice daily results in PSA mean reduction of more than 90 from. Nubeqa is contraindicated in women who are or may become pregnant. Darolutamide is an androgen receptor AR inhibitor.
Therefore NUBEQA is not to be used in women who are or may becomepregnant.
 Frequently Asked Questions About Nubeqa Darolutamide Cancerconnect
Frequently Asked Questions About Nubeqa Darolutamide Cancerconnect
Https S27 Q4cdn Com 906368049 Files News 2019 Zacks Scr Research 08262019 Epix Bautz Pdf
 Fda Approves Bayer S Nubeqa Darolutamide A New Treatment For Men With Non Metastatic Castration Resistant Prostate Cancer
Fda Approves Bayer S Nubeqa Darolutamide A New Treatment For Men With Non Metastatic Castration Resistant Prostate Cancer
 Nubeqa Darolutamide Tablets Uses Dosage Side Effects Interactions Warning
Nubeqa Darolutamide Tablets Uses Dosage Side Effects Interactions Warning
Https 1au3b422k9zdqzddw3my51gg Wpengine Netdna Ssl Com Wp Content Uploads Sites 7 2019 08 Nubeqa S Pdf
 Oncology Drug Reference Sheet Darolutamide Nubeqa Ons Voice
Oncology Drug Reference Sheet Darolutamide Nubeqa Ons Voice
 Nubeqa Darolutamide Clinical Information
Nubeqa Darolutamide Clinical Information
 Darolutamide Delays The Spread Of Some Prostate Cancers National Cancer Institute
Darolutamide Delays The Spread Of Some Prostate Cancers National Cancer Institute
 Prostate Cancer And Non Metastatic Castration Resistant Prostate Cancer Read Slide Landscape Overview Ppt Download
Prostate Cancer And Non Metastatic Castration Resistant Prostate Cancer Read Slide Landscape Overview Ppt Download
Https Www Amcp Org Sites Default Files 2019 12 Mac Nub Us 0020 2 20nubeqa 20presentation 20for 20amcp 20webinar Pdf
 Fda Approves Bayer S Nubeqa Darolutamide A New Treatment For Men With Non Metastatic Castration Resistant Prostate Cancer
Fda Approves Bayer S Nubeqa Darolutamide A New Treatment For Men With Non Metastatic Castration Resistant Prostate Cancer
 Prostate Cancer And Non Metastatic Castration Resistant Prostate Cancer Read Slide Landscape Overview Ppt Download
Prostate Cancer And Non Metastatic Castration Resistant Prostate Cancer Read Slide Landscape Overview Ppt Download
Https Www Amcp Org Sites Default Files 2019 12 Mac Nub Us 0020 2 20nubeqa 20presentation 20for 20amcp 20webinar Pdf

Comments
Post a Comment