- Get link
- X
- Other Apps
Common side effects 20 reported by patients receiving Vitrakvi in clinical trials include fatigue nausea cough constipation diarrhea dizziness vomiting and increased AST and ALT enzyme blood levels in the liver. Annual report and for clinical studiestrials number of patients entered into each studytrial.
 Cancer Drug From German Pharma Giant Bayer Gets Us Approval News Dw 26 11 2018
Cancer Drug From German Pharma Giant Bayer Gets Us Approval News Dw 26 11 2018
Contingent upon verification and description of clinical benefit in confirmatory trials.
Vitrakvi clinical trials. There are ongoing clinical trials moreover including Bayers SCOUT trial and a Childrens Oncology Group-sponsored trial currently evaluating larotrectinib in previously treated and untreated pediatric NTRK fusion-positive cancers respectively though neither are uniquely evaluating the drug in brain tumors. And memory loss were reported in any of the Vitrakvi clinical trials to date. The drug is a histology independent cancer treatment that targets all solid tumours with a certain genetic mutation the NTRK gene fusion.
Have a neurotrophic tyrosine receptor kinase NTRK gene fusion without a known acquired resistance mutation. Explore 377550 research studies in all 50 states and in 220 countries. Submit final reports to this NDA as a supplemental application.
In clinical trials of patients with TRK fusion cancer Vitrakvi demonstrated an ORR of 75. Vitrakvi larotrectinib capsules 25 mg and 100 mg for the. Vitrakvi larotrectinib has provisional approval in Australia for the treatment of adult and paediatric patients with locally advanced or metastatic solid tumours that.
The FDA based its approval on 3 clinical trials that included 55 adults and children with several different cancer types all with NTRK positive tumors. Bayer announced additional clinical trial results for Vitrakvi larotrectinib and latest research on its investigational small molecule aryl hydrocarbon receptor AhR inhibitor BAY 2416964 to. VITRAKVI is an approved drug that blocks the action of the NTRK gene fusion.
Common side effects reported by patients receiving Vitrakvi in clinical trials include fatigue nausea cough constipation diarrhea dizziness vomiting and increased AST and ALT enzyme blood. Vitrakvi is the first treatment to receive a tumor-agnostic indication at the time of initial FDA approval. Larotrectinib sold under the brand name Vitrakvi is a medication for the treatment of cancer.
No further developmental data is currently available. For administrative purposes all. 2 DOSAGE AND ADMINISTRATION 21 Patient Selection Select patients for treatment with VITRAKVI based on the presence of a NTRK gene fusion in tumor specimens see Clinical Studies 14.
It was discovered by Array BioPharma and licensed to Loxo Oncology in 2013. Have a neurotrophic receptor tyrosine kinase NTRK gene fusion without a known acquired resistance mutation are metastatic or where surgical resection is likely to. Larotrectinib was initially awarded orphan drug status in 2015 for soft tissue sarcoma and breakthrough therapy designation in 2016 for the.
This study will enroll adult and paediatric patients suffering from a solid tumor with NTRK gene fusion for whom the decision to treat their disease with VITRAKVI has been made by their treating physicians. The patients tumors could not be removed through surgery or had gotten worse after treatment. Vitrakvi larotrectinib is a kinase inhibitor indicated for the treatment of adult and pediatric patients with solid tumors that.
Risk factors and risk groups Malnutrition prematurityperinatal asphyxiaeg. ClinicalTrialsgov is a registry and results database of publicly and privately supported clinical studies of human participants conducted around the world. An FDA-approved test for the detection of NTRK.
Bayers Vitrakvi larotrectinib will be available via the NHS in England and Wales to treat a variety of cancers. It is an inhibitor of tropomyosin kinase receptors TrkA TrkB and TrkC. Risk is also being evaluated in the contextof the important potential risk severe neurologic reactions.
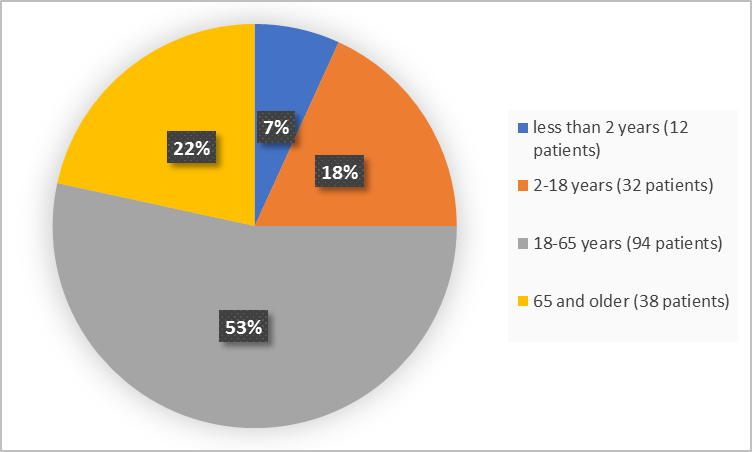 Drug Trials Snapshots Vitrakvi Fda
Drug Trials Snapshots Vitrakvi Fda
 Drug Trials Snapshots Vitrakvi Fda
Drug Trials Snapshots Vitrakvi Fda
 Rozlytrek Vs Vitrakvi How Do These Cancer Drugs Compare
Rozlytrek Vs Vitrakvi How Do These Cancer Drugs Compare
 Bayer Loxo Win Fda Approval For Tumor Agnostic Cancer Drug Vitrakvi
Bayer Loxo Win Fda Approval For Tumor Agnostic Cancer Drug Vitrakvi

 Fda Approves Vitrakvi Larotrectinib The First Ever Trk Inhibitor For Patients With Advanced Solid Tumors Harboring An Ntrk Gene Fusion 1 2
Fda Approves Vitrakvi Larotrectinib The First Ever Trk Inhibitor For Patients With Advanced Solid Tumors Harboring An Ntrk Gene Fusion 1 2
 Basic Information About Larotrectinib Vitrakvi Paginas De Flipbook 1 4 Pubhtml5
Basic Information About Larotrectinib Vitrakvi Paginas De Flipbook 1 4 Pubhtml5
 Vitrakvi Larotrectinib Official Non Us Healthcare Provider Site
Vitrakvi Larotrectinib Official Non Us Healthcare Provider Site
 Basic Information About Larotrectinib Vitrakvi Paginas De Flipbook 1 4 Pubhtml5
Basic Information About Larotrectinib Vitrakvi Paginas De Flipbook 1 4 Pubhtml5
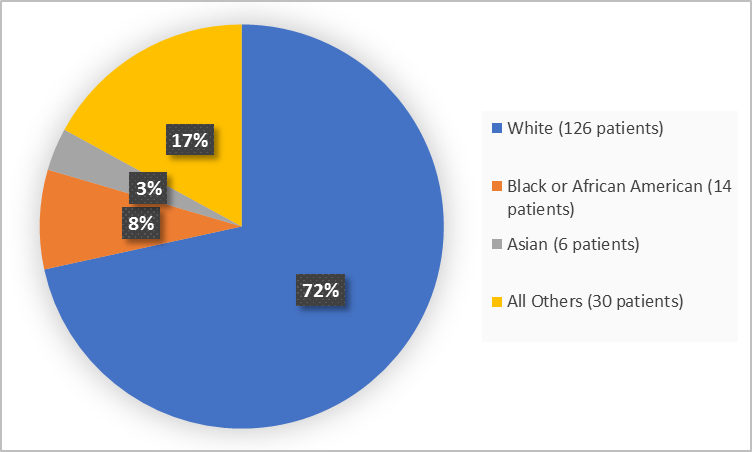 Drug Trials Snapshots Vitrakvi Fda
Drug Trials Snapshots Vitrakvi Fda
 Fda Approves Vitrakvi Larotrectinib The First Ever Trk Inhibitor For Patients With Advanced Solid Tumors Harboring An Ntrk Gene Fusion Intelligence Pharma
Fda Approves Vitrakvi Larotrectinib The First Ever Trk Inhibitor For Patients With Advanced Solid Tumors Harboring An Ntrk Gene Fusion Intelligence Pharma
 Efficacy Data Vitrakvi Larotrectinib
Efficacy Data Vitrakvi Larotrectinib

Comments
Post a Comment