- Get link
- X
- Other Apps
The approval was based on benefits and safety demonstrated in a one-year phase III trial comparing the higher less-frequent dose of the medication with placebo. COPAXONE glatiramer acetate - for solutionsubcutaneous Manufacturer.
 First Generic Version Of Copaxone Approved By Fda To Treat Multiple Sclerosis
First Generic Version Of Copaxone Approved By Fda To Treat Multiple Sclerosis
Currently the Pfizer vaccine has emergency approval from the FDA but full.

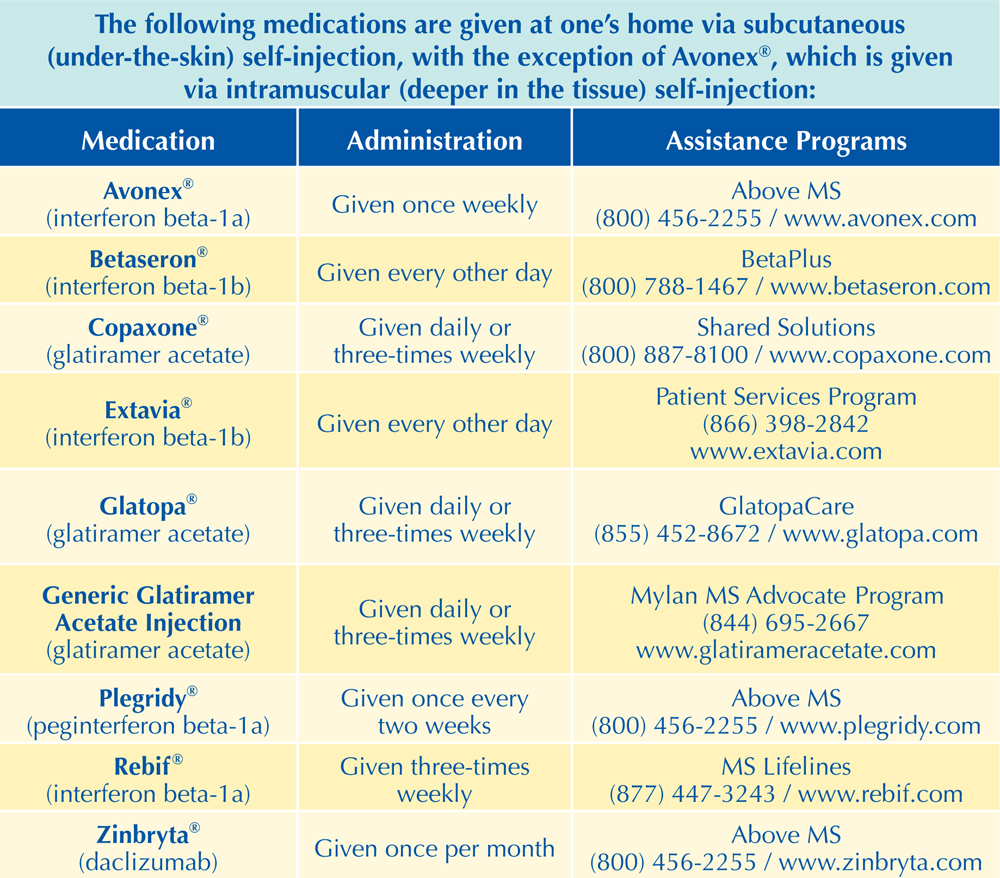
When was copaxone approved by the fda. Medical Devices Cleared or Approved by FDA in 2020. In January 2014 the FDA approved a new 40 mgmL dose of this medication injected three times per week which is double the standard 20 mgmL dose that is injected daily. The FDA granted approval after reviewing the results of the PreCISe study which indicated time to development of a second exacerbation was significantly delayed in patients treated with COPAXONE.
The FDA authorized COVID-19 vaccines too quickly for them to be safe. In this case authorization simply precedes approval similar to how the FDA granted an EUA for the COVID-19 antiviral drug remdesivir six months before it gained FDA approval in October. This new formulation will allow for a less frequent dosing regimen administered by injection for patients with relapsing forms of multiple sclerosis MS.
Food and Drug Administration has approved the first generic form of 40mg glatiramer acetate injection produced by Mylan. Implantable Pulse Generator. First Generic Version of Copaxone Approved by FDA to Treat Multiple Sclerosis April 17 2015 by Patricia Silva PhD In Multiple Sclerosis News News.
Generic version of Copaxone glatiramer acetate injection has been approved by the Food and Drug Administration to treat multiple sclerosis. Pfizer and BioNTech initiated full approval of the Pfizer COVID-19 vaccine from the Food and Drug Administration on May 7. Food and Drug Administration FDA has approved the three-times-a-week COPAXONE 40mgmL a new dose of COPAXONE on Jan 28 2014.
Copaxone is a brand name of glatiramer approved by the FDA in the following formulations. The brand-name drug is produced by Israel-based Teva Pharmaceuticals. THURSDAY April 16 2015 HealthDay News -- The first US.
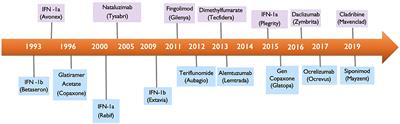
And Teva Neurosciences collectively Teva COPAXONE 40 mgmL product is. Supplemented by Teva in 2013 to receive approval by the FDA of the use of GA 40 mgmL three times per week marketed as COP AX ONE 40 mgmL for the treatment of patients with relapsing forms of multiple sclerosis such as relapse-remitting multiple sclerosis. A new dose of the drug 40 mgmL given subcutaneously will become available in addition to the 20-mgmL daily dose that was first approved by the FDA for this indication in 1996.
Vercise PC and Vercise Gevia Deep Brain Stimulation DBS System - P150031S028. FDA had approved this generic version of the drug in March 2011 because it believed that laboratory data showed that the replica version of the drug was the exact same as the original branded. Generic Copaxone Availability.
TEVA PHARMS USA Approval date. FDA Approves Two New Generic Forms of Copaxone Glatiramer Acetate October 4 2017 UPDATED 1062017 The US. March 15 2014 Peiqing Qian MD.
Food and Drug Administration today approved Mavenclad cladribine tablets to treat relapsing forms of. On April 11 the FDA approved and on June 6 announced changes to the storage requirements for glatiramer acetate injection in prefilled syringes Copaxone Teva Pharmaceutical Industries Ltd and Sanofi-Aventis which allows its storage for up to 1 month rather than 7 days at room temperature. March 29 2019 The US.
First Generic Version Of Teva S Copaxone Cleared By Fda Pmlive
Fda Approves Mylan S Generic Copaxone Pharmatimes
 Two Decades Of Glatiramer Acetate From Initial Discovery To The Current Development Of Generics Sciencedirect
Two Decades Of Glatiramer Acetate From Initial Discovery To The Current Development Of Generics Sciencedirect
 Frontiers Multiple Sclerosis And Cancer The Ying Yang Effect Of Disease Modifying Therapies Immunology
Frontiers Multiple Sclerosis And Cancer The Ying Yang Effect Of Disease Modifying Therapies Immunology
Copaxone Multiple Sclerosis Nanotechnology Products Npd
 Disease Modifying Therapies For Multiple Sclerosis The Bmj
Disease Modifying Therapies For Multiple Sclerosis The Bmj
 Figure 1 From Enduring Clinical Value Of Copaxone Glatiramer Acetate In Multiple Sclerosis After 20 Years Of Use Semantic Scholar
Figure 1 From Enduring Clinical Value Of Copaxone Glatiramer Acetate In Multiple Sclerosis After 20 Years Of Use Semantic Scholar
 Copaxone For Multiple Sclerosis Side Effects Ms News Today
Copaxone For Multiple Sclerosis Side Effects Ms News Today
 Teva Sues Fda Over Bid To Block Approval Of Generic Copaxone
Teva Sues Fda Over Bid To Block Approval Of Generic Copaxone
 The Fda Approved Long Term Treatments For Ms Msaa
The Fda Approved Long Term Treatments For Ms Msaa
Fda Approves Generic Copaxone For Multiple Sclerosis Pharmafile


Comments
Post a Comment